A STORM super-resolution microscope arrived at the Cytology/Imaging Platform of the IJPB Plant Observatory
microscope
superesolution
sFLIM
biphoton
STORM
A cutting-edge microscope, for 3D and multi-color dSTORM super-resolution imaging, allowing spectral fluorescence imaging and photoactivation
Fluorescence microscopy struggles to resolve two distant points by less than 250 to 300 nm. This new microscope, with a spatial resolution of 15 nm, will provide a particularly significant advancement for teams engaged in cutting-edge scientific research, particularly for fine subcellular imaging (plant cell wall nanostructures, intracellular vesicles, actin filaments, microtubules, large protein complexes, chromosomes, etc.), as well as for 3D reconstructions and modeling, where the gain in resolution (in all three axes) is highly substantial.
The imaging platform from the the Plant Observatory PO welcomes a state-of-the-art microscope, for 3D and multi-color dSTORM super-resolution imaging, obtained through the ERC-funded STORMtheWALL project (actu IJPB). This equipment enables the tracking of 3D structures of individual molecules and cellular dynamics and will facilitate the study of plant wall nanostructure, as well as other subcellular structures. The system also includes an sFLIM module (spectral Fluorescence Lifetime Imaging Microscopy) offering capabilities of spectral imaging and fluorescence lifetime imaging under bi-photon excitation. Coupled with the photostimulation module, the sFLIM, will allow simultaneous monitoring and control of multiple biochemical and molecular processes in planta using biosensors imaged by FRET-FLIM after photoactivation. Beyond the ERC project, this equipment, unique in France, will remain accessible and will offer acquisition of images of exceptional quality and resolution (both spectral and in 3D).
The STORM technology involves iterative steps of photoactivation/photobleaching and detection of single molecules. It enables the localization of individual molecules providing an excellent spatial resolution (3D). The sFLIM and photostimulation modules allow simultaneous control and observation of dynamic biochemical and molecular processes in living plants using a photoactivable activator (optogenetics) and multiple FRET biosensors in parallel. Coupled with highly sensitive detectors, the system allows spectral "lifetime" imaging (sFLIM) by photon counting in a spectral range from 430 to 650 nm (16 channels), even in low light conditions. The equipment surpasses typical spatial limits using multi-target optical nanoscopy to visualize cellular processes and then extends beyond their temporal limits using light-controlled sensors and multiplexed intracellular biosensors to disrupt and simultaneously monitor in vivo system's dynamic.
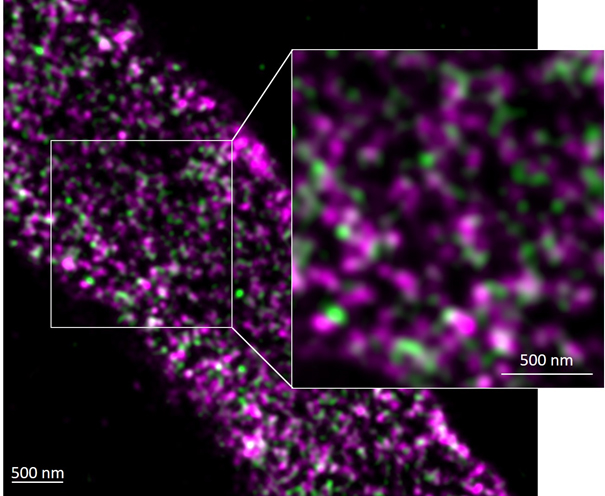
Ultrastructure of Arabidopsis pollen tube plant cell walls as seen by dSTORM immunostained against pRALF4:RALF4-HA (green) and demethylated pectin homogalacturonan (LM19, magenta), ©Kalina T. Haas
This microscope opens a new dimension for understanding nanoscale cellular mechanisms and will provide unprecedented data sets for establishing models of biological processes. These models are essential building blocks of multiscale models predicting crop growth and yield in changing environments.

The imaging platform from the the Plant Observatory PO welcomes a state-of-the-art microscope, for 3D and multi-color dSTORM super-resolution imaging, obtained through the ERC-funded STORMtheWALL project (actu IJPB). This equipment enables the tracking of 3D structures of individual molecules and cellular dynamics and will facilitate the study of plant wall nanostructure, as well as other subcellular structures. The system also includes an sFLIM module (spectral Fluorescence Lifetime Imaging Microscopy) offering capabilities of spectral imaging and fluorescence lifetime imaging under bi-photon excitation. Coupled with the photostimulation module, the sFLIM, will allow simultaneous monitoring and control of multiple biochemical and molecular processes in planta using biosensors imaged by FRET-FLIM after photoactivation. Beyond the ERC project, this equipment, unique in France, will remain accessible and will offer acquisition of images of exceptional quality and resolution (both spectral and in 3D).
The STORM technology involves iterative steps of photoactivation/photobleaching and detection of single molecules. It enables the localization of individual molecules providing an excellent spatial resolution (3D). The sFLIM and photostimulation modules allow simultaneous control and observation of dynamic biochemical and molecular processes in living plants using a photoactivable activator (optogenetics) and multiple FRET biosensors in parallel. Coupled with highly sensitive detectors, the system allows spectral "lifetime" imaging (sFLIM) by photon counting in a spectral range from 430 to 650 nm (16 channels), even in low light conditions. The equipment surpasses typical spatial limits using multi-target optical nanoscopy to visualize cellular processes and then extends beyond their temporal limits using light-controlled sensors and multiplexed intracellular biosensors to disrupt and simultaneously monitor in vivo system's dynamic.

Ultrastructure of Arabidopsis pollen tube plant cell walls as seen by dSTORM immunostained against pRALF4:RALF4-HA (green) and demethylated pectin homogalacturonan (LM19, magenta), ©Kalina T. Haas
This microscope opens a new dimension for understanding nanoscale cellular mechanisms and will provide unprecedented data sets for establishing models of biological processes. These models are essential building blocks of multiscale models predicting crop growth and yield in changing environments.


Back

Références:
> S Moussu, H K Lee, K T Haas, C Broyart, U Rathgeb, D D Bellis, T Levasseur, S Schoenaers, G S Fernandez, U. Grossniklaus, E Bonnin, E Hosy, K. Vissenberg, N Geldner, B Cathala, H Höfte & J Santiago. Plant cell wall patterning and expansion mediated by protein-peptide-polysaccharide interaction. Science 382, 719–725 (2023), DOI : https://doi.org/10.1126/science.adi4720
actu IJPB & communiqué de presse INRAE, 10/11/23
> K T Haas, R Wightman, E M Meyerowitz & A Peaucelle. Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science (2020), DOI : https://doi.org/10.1126/science.aaz510
actu IJPB & communiqué de presse INRAE, 10/11/23
> S Moussu, H K Lee, K T Haas, C Broyart, U Rathgeb, D D Bellis, T Levasseur, S Schoenaers, G S Fernandez, U. Grossniklaus, E Bonnin, E Hosy, K. Vissenberg, N Geldner, B Cathala, H Höfte & J Santiago. Plant cell wall patterning and expansion mediated by protein-peptide-polysaccharide interaction. Science 382, 719–725 (2023), DOI : https://doi.org/10.1126/science.adi4720
actu IJPB & communiqué de presse INRAE, 10/11/23
> K T Haas, R Wightman, E M Meyerowitz & A Peaucelle. Pectin homogalacturonan nanofilament expansion drives morphogenesis in plant epidermal cells. Science (2020), DOI : https://doi.org/10.1126/science.aaz510
actu IJPB & communiqué de presse INRAE, 10/11/23