How To turn his loss into a win during complex genome species evolution?
Could duplicated genes loss be an evolutionary driving force as genes duplication do? Studiing rapeseed reproduction, the team of Eric Jenczewski from "Meiosis Mechanisms" show that, far from beeing deleterious, loss of a duplicated copy of a main gene involved in chromosomes exchanges during meiosis could give an adaptative advantage. Published in Nature Communications, these results open up new perspectives in plant breeding, in particular to select new grown species harboring several genomes as for example triticale, hybrid between wheat and rye.
All flowering plants had their genomes duplicated at least once during evolution (polyploidy events). This evolutive success is not obvious becauce presence of more than two complete sets of chromosomes, often originating from different species, lead to errors during meiosis. Since decades, scientists try to understand how nevertheless diploid species succeed to transmit their chromosomes to their progeny during this crucial step of reproduction. Research of the team leaded to consider an answer surprisingly simple : to eliminate one duplicated copy of essential genes for meiotic recombinaison.
Always alone !
Scientifics from the team « Meiosis Mechanisms » were interested in evolution of genes coding a protein involved in genetic exchanges between chromosomes : MSH4. They showed that the genes coding the MSH4 protein came back in a unique copy form following polyploidy events during history of flowering plants. Only the more récent polyploids recent polyploids as wheat, cotton plant or rapeseed harbore several copies. Surprised by this systematic loss of duplicated genes during a so short period, scientists studied consequencies of loss of one of MSH4 copy in rapeseed where two are present.
Waste without consequence
Simultaneous inactivation of the two copies of MSH4 in rapeseed lead to a drastic decrease of the number of genetic exchanges between chromosomes, confirming the essential role played by this protein in meiotic recombination in this species. Conversely, the loss of one of the 2 copies had no consequences on the successful completion of meiosis in this species. Only one functioning copy (allele) to ensure an equilibrated chromosomes segregation during meiosis is necessary.
Less to be, better it is !
It turns to be different, if we are interested in genetic exchanges which can happen between chromosomes inherited from the parental species of rapeseed. Their number is proportional to the number of MSH4 functional copies : it is maximal when the two copies are functional, and decrease progressively with the accumulation of deleterious alleles in the plant for one or the other copy, and it is close to nothing when the two copies are inactivated. Far from beeing deleterious, this loss of duplicated copy could be beneficial, limiting risks of illegitime exchanges perturbating the segregation of colza chromosomes. These works open the way to the selection of new cultivated species combining genomes of several species, as it was done for tritical.
Financial support (PhD) by the european training network COMREC.
Back
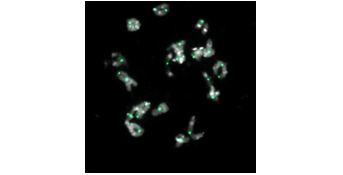
Immunocytologic labeling of genetic exchanges between chromosomes (green foci) in a rapeseed plant presenting a unique functional allele MSH4
Communiqué de presse INRA 06/06/19
Contact:
Eric Jenczewski (01 30 83 33 08)
Institut Jean-Pierre Bourgin (INRA, AgroParisTech)a
Associated Division:
Plant Biology and Breeding
Associated Centre:
Ile-de-France-Versailles-Grignon
Contact(s) press:
INRAE Press room (01 42 75 91 86)
Référence:
Reducing MSH4 copy number prevents meiotic crossovers between non-homologous chromosomes in Brassica napus.Adrián Gonzalo, Marie-Odile Lucas, Catherine Charpentier, Greta Sandmann, Andrew Lloyd et Eric Jenczewski. Nature Communications. 29 mai 2019.
DOI : orcid.org/0000-0001-7821-5384