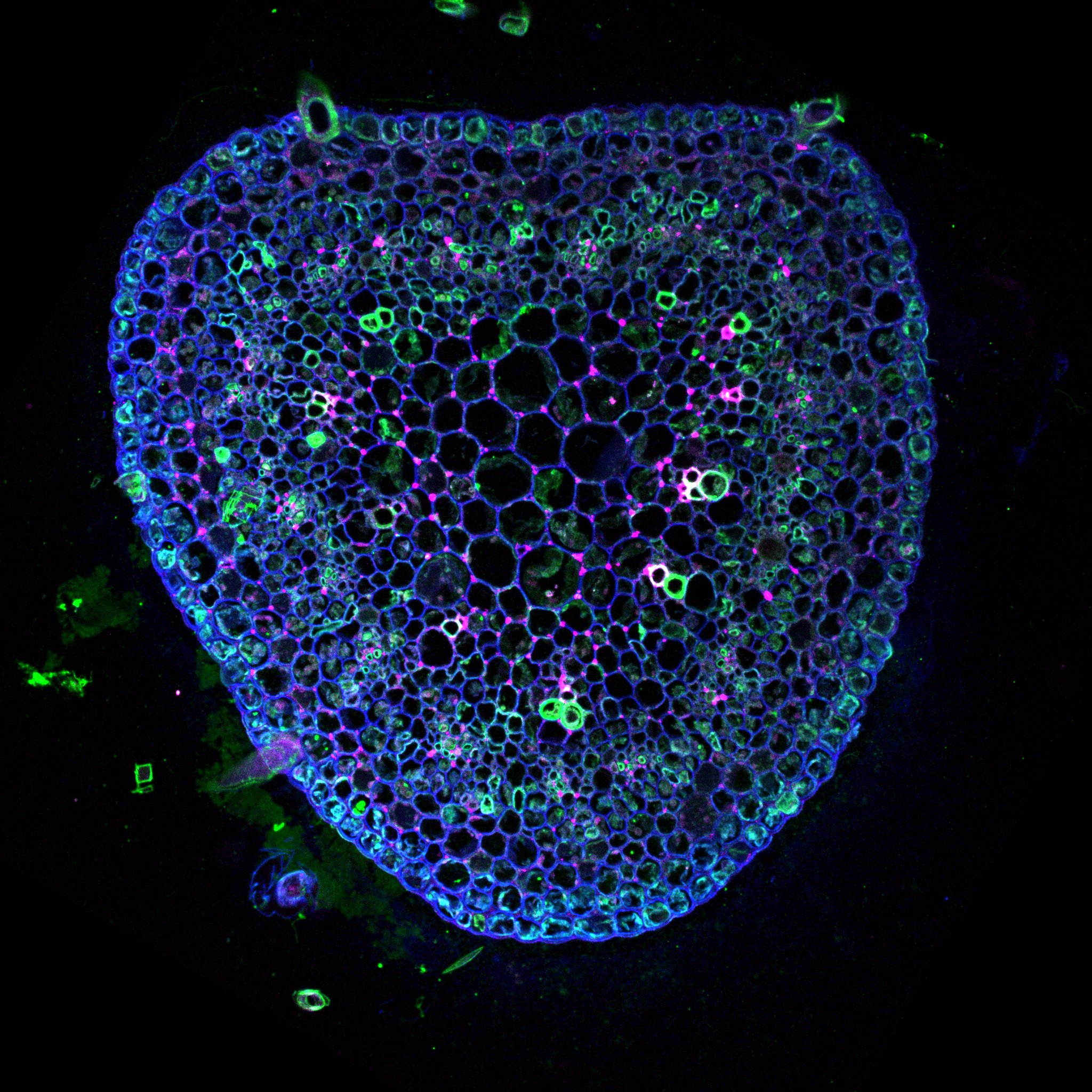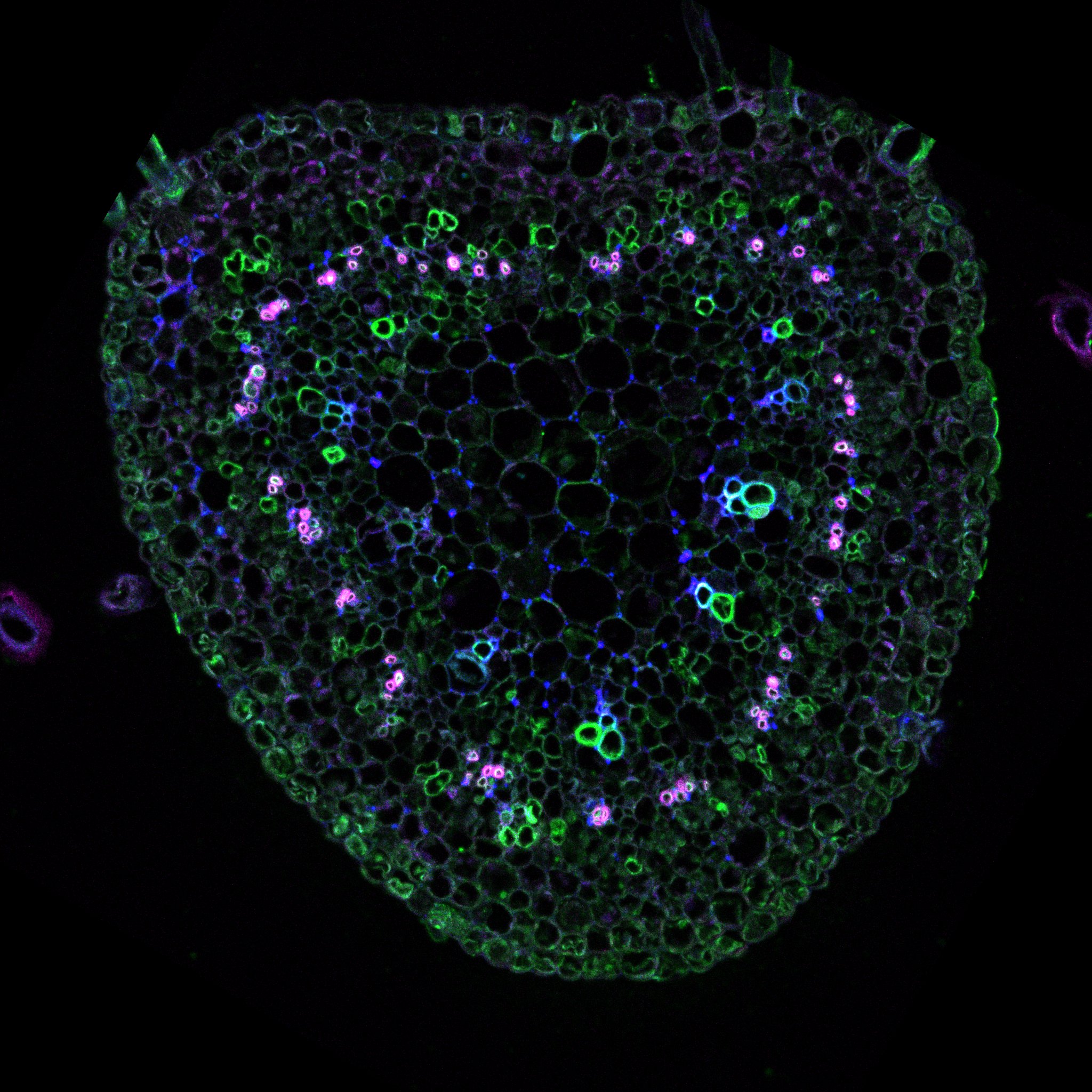
The morphogenesis in plants is a fascinating phenomenon: plant shape is a results of genetic and environmental cues generating beautiful geometry that could be described mathematically.
The golden angel of the position of the lateral organs (Phyllotaxis) is one example (http://www.lps.ens.fr/~douady/PhyllotaxisIndex.html; https://www.youtube.com/watch?v=FOuoJVZqUV8 ) another example are the lobes of pavement cells (https://www.botany.one/2014/02/pavement-cells-living-puzzle/ , link to paragraph).
Ultimately, if you want to understand morphogenesis you need to untangle the mystery of plant growth. A mystery crucial for humanity which has been cultivating different crops for thousands of years.
Since the Neolithic farmers humanity have been selecting plants based on their growth capacity to maximize the yield. Competing theories for the growth process are presented regularly but none of them manage to present a convincing complete vision of this process.
Were is the mystery of plant growth?
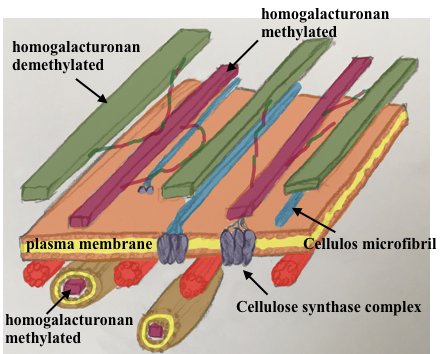
The plant cell is surrounded by a very dense extracellular matrix called the cell wall. This structure could get extremely rigid as observed in wood. Although it is the very first cytological element observed under the microscope and was used for to define the term “cell” (REF to the book) we are far from understanding its chemistry and organization. It is constituted of polymers of sugars associated with different proteins. The most know and by far the most studied is the cellulose. Cellulose was the first biopolymer to be isolated and it is still crucial for the industry. The cell wall contains also other polymers key for the growth process, e.g. the pectin, which we will explore in the following sections. 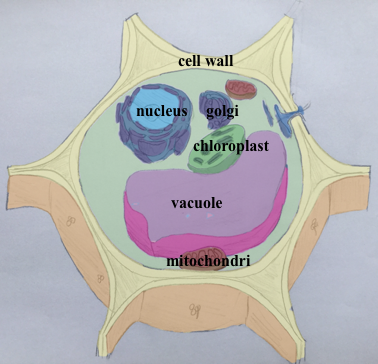
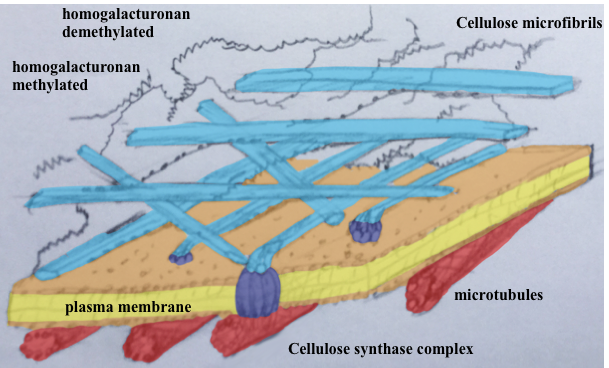
In order to grow, plant cell needs to stretch its cell wall. To do so, cell uses pressurised water (the turgor pressure) achieved through increased ionic concentration inside the cell. This stretching is then transformed into growth through cell wall loosening and remodelling. The first mystery lies in the fact that this has to happen without the cell exploding. The second mystery is that the turgor pressure is isotropic whereas plant morphogenesis is based on the oriented (anisotropic) growth.
Next, we need to figure out at which scale to work in time and space. Growth is both extremely fast and extremely slow: for us humans, the plant growth is too slow. We need accelerated films to visualize it (plantsinmotion.bio.indiana.edu/). Those movement exposes the regulation processes involved in morphogenesis and how they dependent on the environment, internal and external mechanical stimuli. The scale of the study is then the whole organ, which is based on the coordinated cellular growth (http://www.msc.univ-paris-diderot.fr/spip.php?rubrique381 ). On the other hand, the molecular processes underlying the growth are happening at a very fast rate (milliseconds) and exert spatially confined effects (nanometr).
My work focuses on the finding the exact mechanism of growth: which chemical or mechanical elements trigger growth and which are the effectors. For this we work at the organ, tissue, cellular and sub-cellular levels. We work simultaneously on the chemistry and the mechanics of the tissue in combination with the genetic regulation. From this observations I hope to build up a model of plant morphogenesis. Ultimately this should lead to new methods to manage the plant growth for the best benefits of humanity and in respect of the environment.
bottlenecks
-The first one is due to the instantaneous reaction of the living organisms to the extracellular matrix changes. A series of interrelated regulatory feedback loops integrate the redundant function of the chemical components of the extracellular matrix. The classic mutagenesis approach is thus inefficient to study its biology as all changes are corrected in real-time to maintain the biological function of cells. To lift this limitation, we created inducible transgenic plants permitting to control in time and space changes in cell wall chemistry.
-The major limitation to understanding the biology of extracellular matrix is the absence of microscopic techniques adapted to its study. Understanding in situ biopolymer function requires resolving simultaneously the chemical and the spatial organization of the polymers with a nanometer resolution. In collaboration with Kalina Haas introduced super-resolution microscopy to plant cell wall science permitting ultrastructural imaging of the plant cell walls with biochemical contrast. They developed a technique for the quantitative imaging of targeted polymers and their three-dimensional structure directly in the cell wall.
-The final bottleneck is more conceptual and is linked to the complexity of the growth process that combines the biological, chemical, and mechanical components. To handle all these aspects, we need to work at the interface between different scientific disciplines thanks to the collaboration, and more importantly, mobility between different laboratories. With this, we are bring to our work fresh innovative thinking and put aside dogmas. This work sparked the paradigm shift that opened new horizons for understanding the fundamentals of plant physiology, but also the study of the extracellular matrix as an active component of biology in general.
1. Measuring the growth
The first step of each new study on morphogenesis is to develop the least invasive method to measure the growth. I have participated in the development of various technics to study root, hypocotyl and leaf. Each time it is a different challenge.
Once the images are taken the important part is to measure the growth at the smaller possible scale and work as close as possible to physiological condition; bring the resolution closer and closer to the molecular level and stile have a global view of the processes.
We are collaborating with Julin Derr (http://www.msc.univ-paris-diderot.fr/spip.php?rubrique150 http://www.msc.univ-paris-diderot.fr/spip.php?rubrique154 ) to generate the ultimate imaging process that will manage extraction of 3D growth proses of full organ in physiological condition. In addition it will be analysed automatically in terms of locale tissue growth/compression.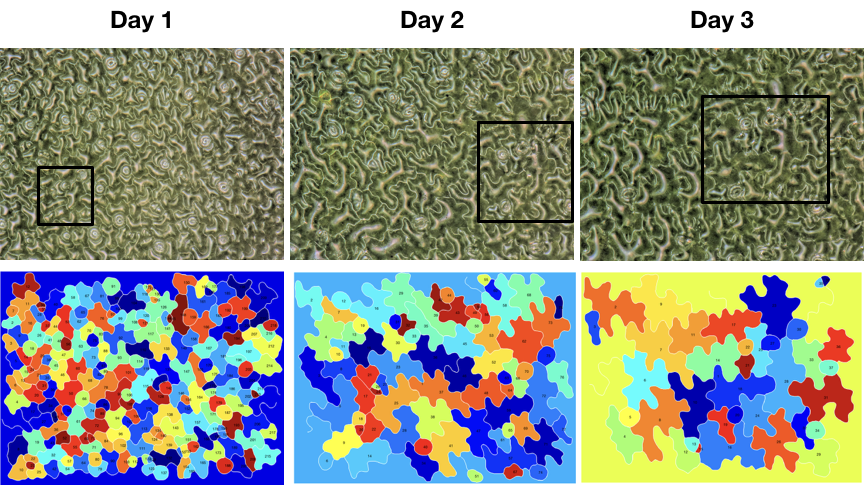
2. Phylotaxis
Plants lateral organs (e.g. leafs on the stem) are deposed following a mathematical regularity (https://www.youtube.com/watch?v=QPB-j2O9J6I). This pattern is generated at the apex of the plant were new lateral organ appears in a very organized way in time and space. The golden angel deposition of the lateral organ is very common and could be found in the fossil records as early as in the pre carboniferous.
My work with Yves Couder (https://www.academie-sciences.fr/fr/Liste-des-membres-de-l-Academie-des-sciences-/-C/yves-couder.html https://fr.wikipedia.org/wiki/Yves_Couder) demonstrated that the deposition of the lateral organ in a spiral (phyllotaxis) is observed in all the apical growth processes. It has occurred independently at least three times during the evolution: in green plants, red and brown algae.
Our final project, which is currently at a hold, was to explore the spiral organization of tip-growing coral. We planned to test if the spiral organization could be used to evaluate the health of corals in situ, a powerful tool to predict/prevent coral extinction.
I also worked on the genetic regulation of the growth that in Arabidopsis thaliana, a model plant in research permits to conserve the inherent organization appearing at the apex of the plant. We showed that the proper spatial expansion of a cell wall modifying enzyme (a pectin methyl-esteras) was crucial in this proses. This was our first observation, notably that changes in the methylation of a component of the cell wall could be a key element in a growth of cell.


3. Organ formation as a biomechanical proses
The origin of the phyllotaxis pattern at the apex is based on a local increase in the cell growth at the position of future lateral organs. This was known to be regulated through hormonal (local accumulation of auxin, https://www.sciencedirect.com/science/article/pii/S0960982217306358#fig3 ) and chemical modification of cell wall characteristic (expansins). We demonstrated that modification of one component of the cell wall (the pectin, specifically the homogalacturonans) by the demethylation of a carboxyl group on the galacrturonane sugar was both necessary and sufficient to generate organ formation. I also demonstrate that his chemical change is under the control of local accumulation of the phytohormon auxin and simultaneously feeds-back to its spatial accumulation. This revels the chemical, hormon and potentially mechanical loop behind the spatio-temporal regulation of organ formation and ultimately the phyllotaxis.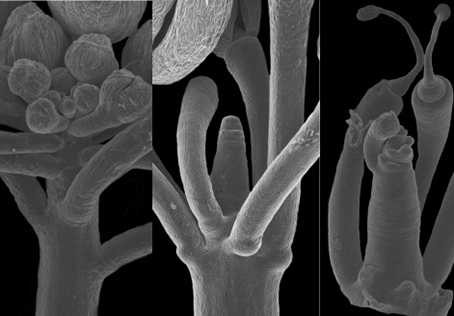
4. Atomic Forms Microscopy: relation between cell wall elasticity and growth
How could a simple modification of a chemistry in the cell wall be so pivotal in the organ growth? Our first attempt to answer this question required the adaptation of the AFM to plant tissue (https://www.youtube.com/watch?v=tcelarWxT7A ). We developed an approach permitting to measure tissue and cellular mechanical characteristic of plants. This was later used to study plant and animal morphogenesis.
In plants, to our astonishment, the reduction in elasticity of the cell wall correlates with changes in the growth. This was observed at the tissue and organ level. We published work on meristem, hypocotyl, roots, leaf, stem end wood. A strong correlation between growth, softening of the tissue elasticity (and not viscosity) was systematically found.
We observed that the asymmetric cell wall loosening (due to changes in pectin methylation) correlates and predict anisotropic cell expansion. Thus anisotropic expansion could be generated not through cell wall anisotropy but more due to cell mechanical polarity.
But this is to simple: in our last study on the effect of cellulose synthase deficiency on the meristem function we revealed that such defect is leading to cell wall reduced elasticity but not an increase in cell growth. Thus the link to growth is not systematic and other parameters have to be taken into account. 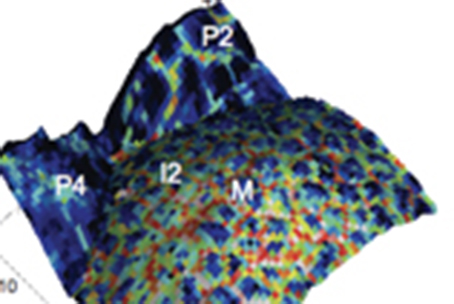
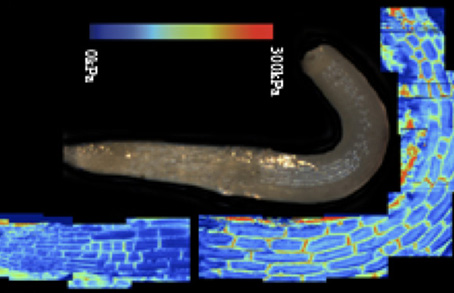
5. Sonocythology: the growth-related vibrations and cell wall mechanics (are growing cells singing?)
The correlation between cell growth and elasticity of the cell wall is contrary to mechanical concept: growth is an irreversible modification whereas elasticity is per definition reversible process. To understand this discrepancy, we started a new project which explores the possibility that rapid mechanical stimulation could be generated by cells to probe locale elasticity and stimulate locale growth through increased exocytosis. This hypothesis is compatible with recent founding that plants are sensitive to sound (https://www.nationalgeographic.com/science/2019/01/flowers-can-hear-bees-and-make-their-nectar-sweeter/ DOI 10.1007/s00442-017-3862-z). We explore this by using AFM to record tissue oscillation and resonant confocal microscopy to measure mechanically stimulated exocytosis.
6. Pavement cells math and music
Originally we explored the morphogenesis of pavement cells to question the turgor driven stress-induced morphogenesis in plants. These cells form protrusion generating a beautiful pavement of puzzle shaped cells. We performed a mathematical analysis of the evolution of the lobes and shape of the cells. We observed that the lobes of cells have a similar structure to the sound generated by a musical instrument: a fundamental oscillation and the overtone series with exponential decrease in the intensity. This similarity reveals the extremely regulated pattern that is generated by the cells and could be heard by listening to the shape of cells (Click hire). Yes, it is not as beautiful as a real instrument but it is astonishing nevertheless.
As an art project, we are now exploring how using the cell shape as a generator of melodies and rhythms we could explore the intrinsic beauty of plants through our more emotional, musical brain. We are now showing this to the public.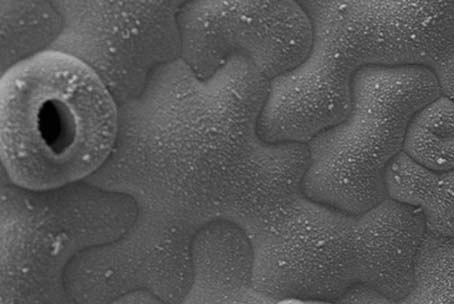
7. Seeing the polymer: the cell wall chemical organization revealed through super resolution microscopy:
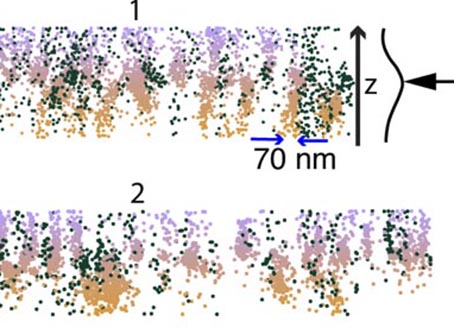
So far, the polymer organization of the cell wall was revealed through electron microscopy. However, this wonderful technique shows the structure but not the chemistry of the polymer. The chemistry is observed through cutting into smale sugars, then the polymer structure is of course lost. In order to create high resolution structural map of the cell wall at the scale of tenths of nanometres, we performed multicolour 3D super resolution imaging (dSTORM) (https://www.youtube.com/watch?v=_sn9OgcQkSw). Such chemical super resolved imaging (chemistry revealed through immuno labelling) revealed that the pectin polymers do not form amorphic gel but rather form nano columns aligned parallel one to the other in a manner very similar to the cellulose. Currently, we are pushing this technique to perform cartography of the polymer structure of the cell wall in different plant’s. Our goal is to generate a new map of cell wall structure and chemistry based on our previous work on meristem. We hope to reveal unexpected new properties for each chemical component.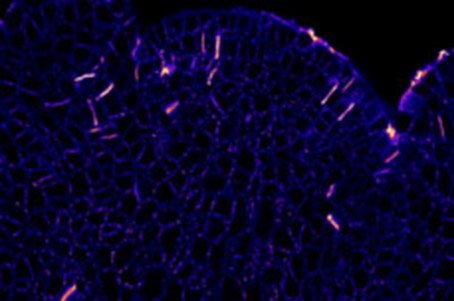
8. “The expending brick”, a new theory of growth
Could we say how growth is working then?
We are now trying to combine all the results in a comprehensible simple model of growth in plants that could explain all the observed phenomena. For this, we need to put aside the stress driven growth model and instead we propose “the expending brick model”. We suppose for a start that growth is an intrinsic property of the cell wall. It is generated by the expansion of the pectin homogalacturonan polymers subsequent to their chemical modification in the cell wall (demethylation). We tested the predictive power of this model using nonlinear finite element modelling (FEM). Applied to pavement cells this model predicted quite accurately cell growth, lobe formation, cell wall thickness and cell wall internal stress.
To explore the limits of this simplistic model we are now planning to expand the model to the tissue level of hypocotyl leaf and eventually meristem.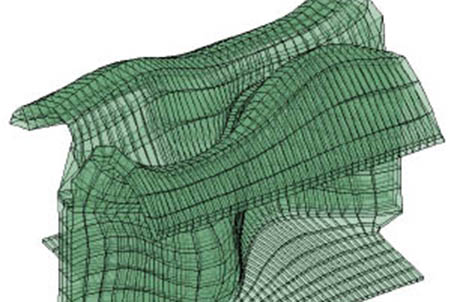
conclusion
With the new model of growth, we are rewriting the paradigm of plant growth. New important parameters of growth are revealed that will serve as a base of our study of the regulatory proses of growth. Breading and selection could be reoriented. For a start we hope to develop a new method of monitoring the growth, which is predictive and non-invasive and could be used in agriculture to detect nutritional defects, predict the yield and direct for ultimate use of fertiliser to minimal environmental impact.
We also hope that the expanding brick could be a good building block for a translational research and will help develop self-expanding or healing artificial tissues.
Finally, the extracellular matrix is also important in the development of animal tissue and during tumorigenesis. A study of extra cellular matrix mechanics and shape could propose new methods for neuronal regeneration or prevention of metastasis