1) Dynamics of Lipid Droplets: Sabine d'Andréa, Isabelle Bouchez, Carine Deruyffelaere.
In dry seeds, the lipid reserves stored in LDs remain stable for several years thanks to the major proteins which surround them, oleosins. When seeds germinate, they mobilize their reserve lipids to allow the seedling to grow. We wanted to understand how the LDs, remarkably stable in the dry seed, could be rapidly degraded during germination. We have shown that the degradation of oleosins precedes the degradation of reserve lipids. Oleosins are labeled for degradation by modifications called ubiquitinations, then extracted from LDs and then degraded by proteolysis. We then wanted to understand how proteins as anchored to LDs as oleosins can be extracted from the surface of CLs. We thus discovered that a protein called PUX10 is necessary to correctly extract the ubiquitinated oleosins from the surface of CL in Arabidopsis seedlings. PUX10 is localized on LDs and binds to ubiquitinated oleosins. It also interacts with an ATPase called CDC48A, known to selectively extract misfolded proteins from the endoplasmic reticulum. PUX10 is thus an adapter protein which recruits this ATPase on the LDs thereby promoting the extraction of ubiquitinated oleosins before their proteolytic degradation. We propose that PUX10 and CDC48A are the core of a new degradation machinery associated with LD, that we have named LDAD (Lipid Droplet-Associated Degradation) (Deruyffelaere 2018, 2015).
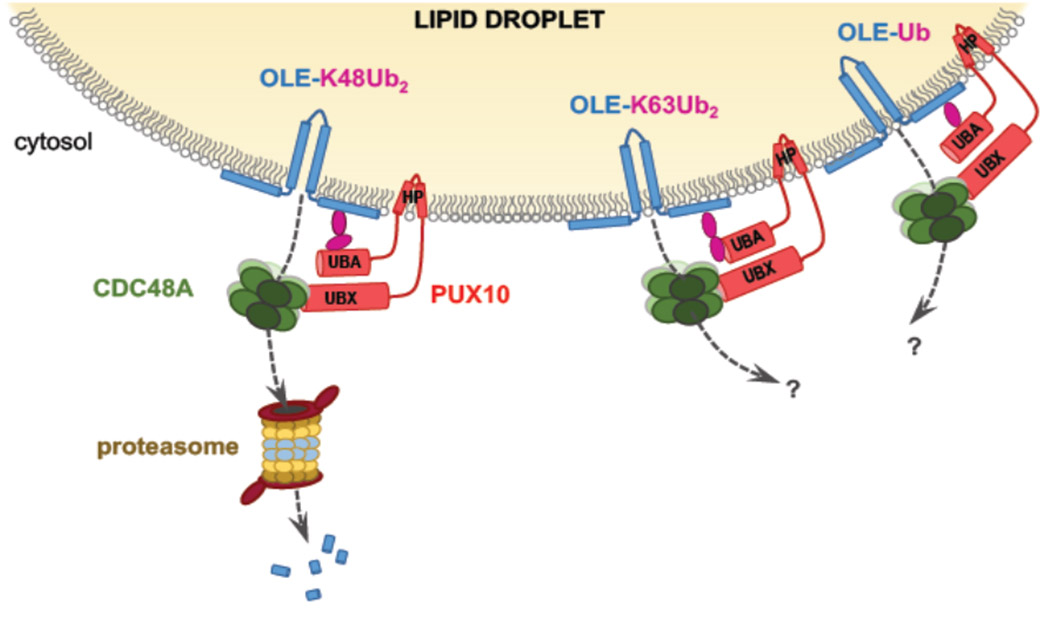
Our results also strongly suggest the existence of CL subpopulations.

Our results also strongly suggest the existence of CL subpopulations.
2) Topology and relative accessibility of proteins associated with plant LDs (Christelle Louis-Mondésir, Thierry Chardot, Yann Gohon).
Oleosines are proteins that are hardly soluble in aqueous medium, and they are inserted on the surface of LDs in a hemi-membrane of phospholipids, which is exceptional in nature. The study of their structure can only be carried out in the presence of surfactants to keep them soluble, or else in situ in their natural environment, LDs.
Our close partnership with the SOLEIL synchrotron (DISCO beamline, St. Aubin) made it possible, using circular dichroism by synchrotron radiation (SRCD), to study the fold of the major oleosin of seed LDs in its natural environment. Within the LDs, the major oleosin is mostly folded, and in an original way with beta strands. To precisely define how this protein was inserted on the surface of LDs, we have developed a structural proteomics approach with the PAPSSO proteomics platform (Le Moulon) and the Metrology beamline of the Synchrotron SOLEIL. Synchrotron radiation has produced hydroxyl (• OH) radicals by photolysis of water. • OH have a very short lifespan (~ μs) and make it possible to oxidize amino acid residues accessible to water. Proteomics permitted to identify, in purified LDs and at the amino acid scale, the regions of the protein that are oxidized and therefore in contact with water. By deduction, the non-oxidized regions are not accessible to water and are therefore at the heart of the neutral lipids of LD.
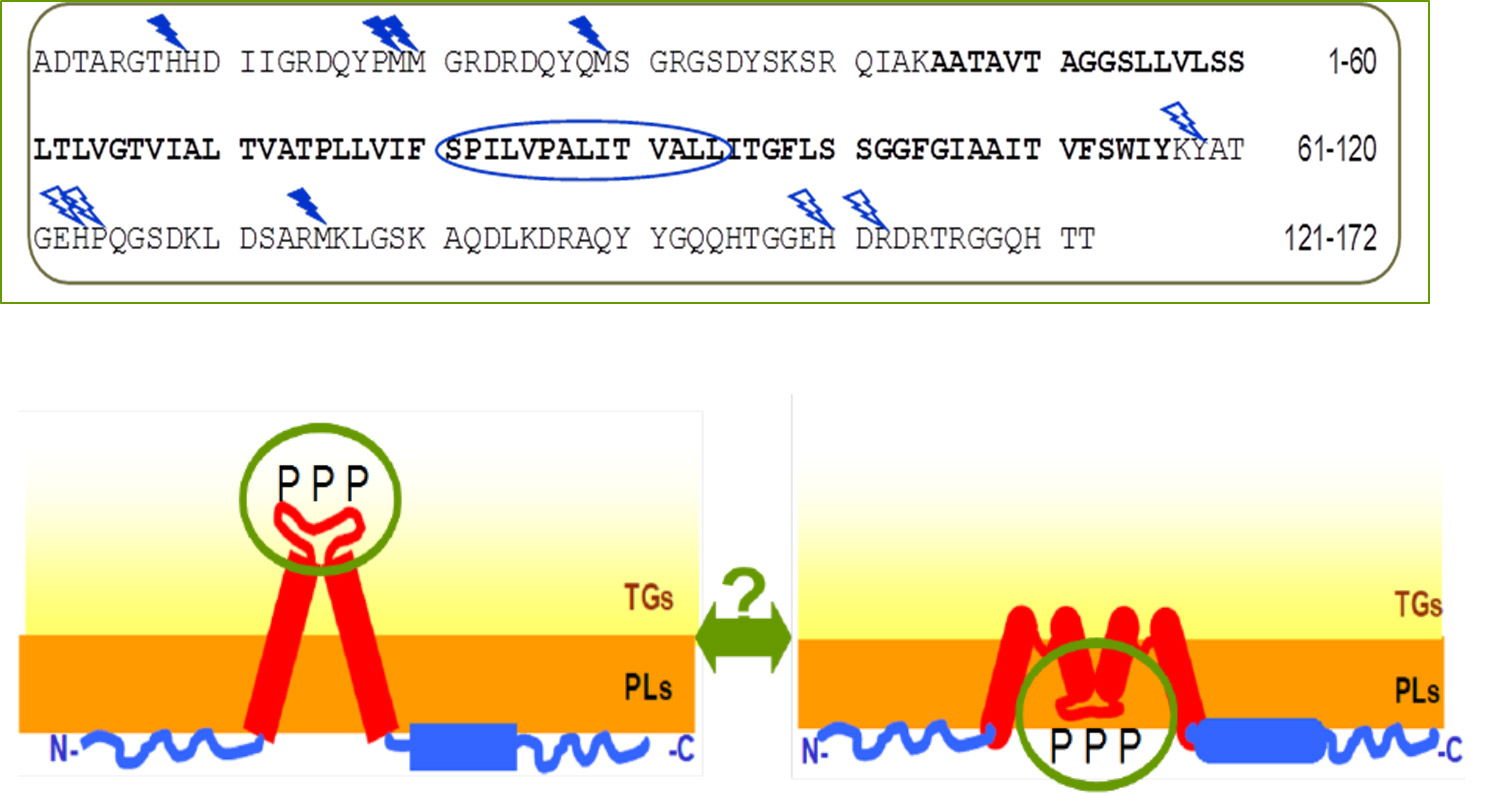
This first study of structural proteomics on intact organelles permitted to determine the topology of oleosin S3 in its natural environment (Baud 2017, Jolivet 2017).
Our close partnership with the SOLEIL synchrotron (DISCO beamline, St. Aubin) made it possible, using circular dichroism by synchrotron radiation (SRCD), to study the fold of the major oleosin of seed LDs in its natural environment. Within the LDs, the major oleosin is mostly folded, and in an original way with beta strands. To precisely define how this protein was inserted on the surface of LDs, we have developed a structural proteomics approach with the PAPSSO proteomics platform (Le Moulon) and the Metrology beamline of the Synchrotron SOLEIL. Synchrotron radiation has produced hydroxyl (• OH) radicals by photolysis of water. • OH have a very short lifespan (~ μs) and make it possible to oxidize amino acid residues accessible to water. Proteomics permitted to identify, in purified LDs and at the amino acid scale, the regions of the protein that are oxidized and therefore in contact with water. By deduction, the non-oxidized regions are not accessible to water and are therefore at the heart of the neutral lipids of LD.

This first study of structural proteomics on intact organelles permitted to determine the topology of oleosin S3 in its natural environment (Baud 2017, Jolivet 2017).
3) Functional and structural information on the functioning of the DGATs of different families (Frank Jagic, Christelle LouisMondésir, Sihem Hentati, Thierry Chardot, Pierre Briozzo)
The Kennedy pathway allows stable storage of fatty acids in eukaryotes in the form of triacylglycerols (TAG). Three membrane acyltransferases catalyze the stereospecific and successive addition of fatty acids to a glycerol backbone. DGATs (diacylglycerol acyltransferases) add the final fatty acid in position sn-3. More than 130 fatty acids can be incorporated into vegetable oils, which gives them specific physical, chemical and nutritional properties. Due to their impact on oil yield and quality, DGATs are prime targets for oil engineering. They belong to 3 distinct phylogenetic families. The first two, mainly expressed in seeds at the endoplasmic reticulum, are involved in the accumulation of oil.
DGAT3s were identified for the first time in 2006. Their localization in the cytosol suggests that these enzymes should be soluble, and be part of an alternative pathway for the synthesis of TG and. DGAT3 is therefore an original target for metabolic, enzymatic and structural studies.
Careful examination of the protein sequence showed a C-terminal ferredoxin-like region conserved in plant DGAT3s. This region is potentially associated with a 2Fe-2S cluster. The purified recombinant protein AtDGAT3 had a brown-red color, fading over time. Thanks to Professor Fontecave's laboratory (Collège de France, Paris), we were able to purify this protein without oxygen and demonstrate using paramagnetic electromagnetic resonance that it contained a cluster [2Fe-2S]. It is the first time that a DGAT has been shown to possess have a [Fe-S] center.
![AtDGAT3 contains a [2Fe-2S] cluster](https://ijpb.versailles.inrae.fr/uploads/2020/03/20/figure3.jpg)
DGAT3s were identified for the first time in 2006. Their localization in the cytosol suggests that these enzymes should be soluble, and be part of an alternative pathway for the synthesis of TG and. DGAT3 is therefore an original target for metabolic, enzymatic and structural studies.
Careful examination of the protein sequence showed a C-terminal ferredoxin-like region conserved in plant DGAT3s. This region is potentially associated with a 2Fe-2S cluster. The purified recombinant protein AtDGAT3 had a brown-red color, fading over time. Thanks to Professor Fontecave's laboratory (Collège de France, Paris), we were able to purify this protein without oxygen and demonstrate using paramagnetic electromagnetic resonance that it contained a cluster [2Fe-2S]. It is the first time that a DGAT has been shown to possess have a [Fe-S] center.
![AtDGAT3 contains a [2Fe-2S] cluster](https://ijpb.versailles.inrae.fr/uploads/2020/03/20/figure3.jpg)
Projects 1 and 3 were supported by the Procope Program, Campus France “Futuroil” 2018-2019, coordinated by T Chardot, I Feussner (Göttingen)
4) Methionine Gamma lyase: P Briozzo, F Jagic, D Machover (Paul Brousse Hospital).
Methionine deprivation causes growth arrest and cancer cells death. To achieve this in vivo we can use the enzyme methionine gamma lyase (MGL). Therapeutic trials using MGL from Pseudomonas putida have been stopped due to its immunogenicity. The MGL of the cheese bacterium brevidacterium aurantiacum is unlikely to be immunogenic. We are trying to increase the stability of the recombinant enzyme for anticancer use
Leader:
Thierry Chardot