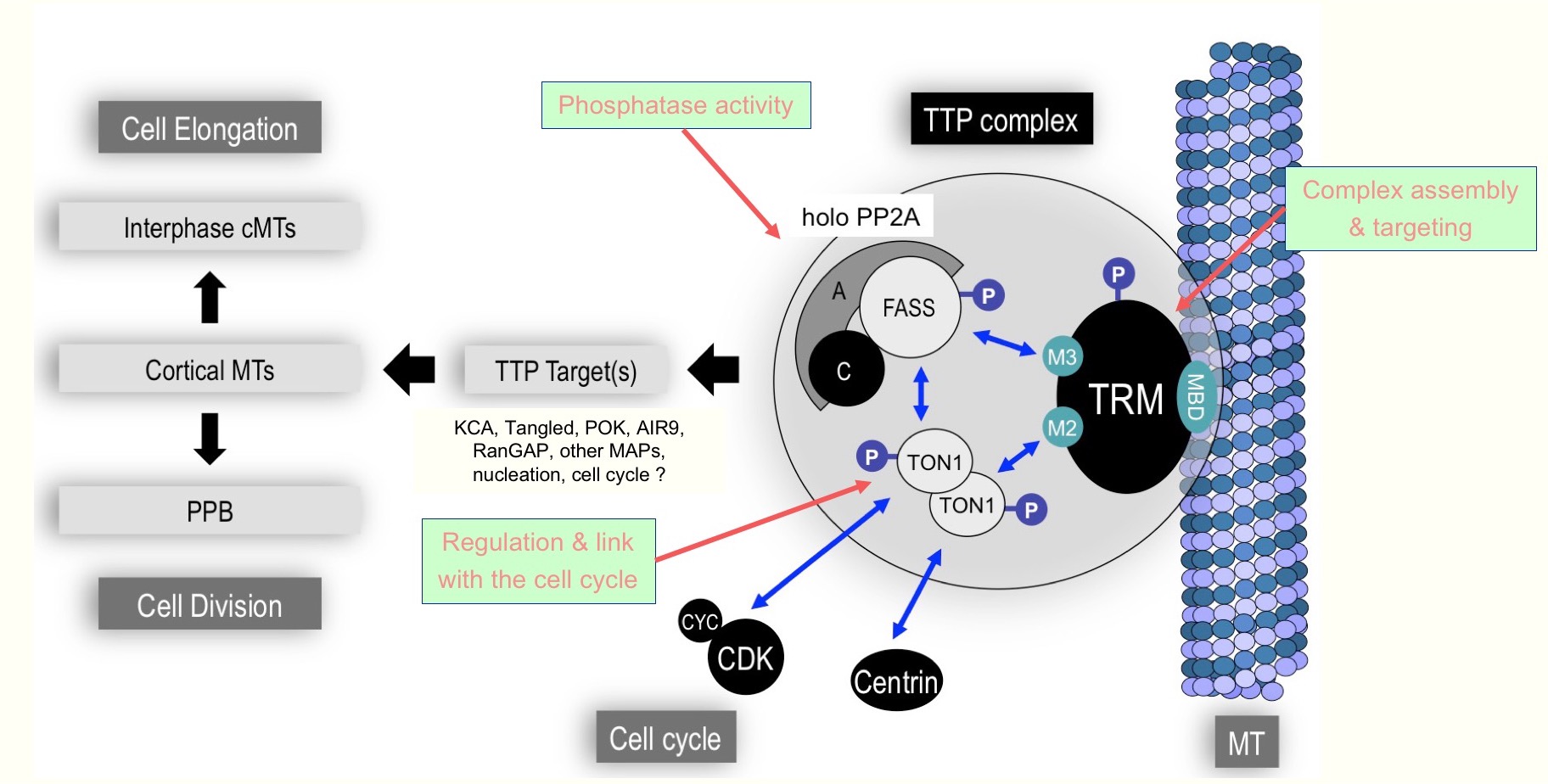
The TTP complex: a network of proteins regulating the organization of the cytoskeleton, the orientation of division and cellular growth
In the course of our studies, we have identified a large protein network, the TTP complex, controlling the spatial arrangement of cortical MTs both in interphase and at the PPB stage.
The preprophase band (PPB) which consists of microtubules and actin filaments is a land-plant specific cytoskeletal array contributing to the pre-mitotic programming of the position of final cytokinetic plane.
This protein complex not only provides an excellent and unique entry point to better understand transitions of the cortical MT cytoskeleton, but it also allows us to explore regulatory circuits connecting these transitions to the cell cycle.
Over the years, most of our activity has been devoted to the characterization of TTP components like TON1 [1], FASS/PP2A [2, 3] or TRM [4]. Altogether, a remarkably consistent picture of this large protein complex emerges from a number of cytological, molecular, genetic and biochemical evidences.
Mutations in core-TTP components like TON1, FASS or PP2A A and C subunits lead to complete absence of PPB and profound disorganization of division planes in Arabidopsis [1, 2, 3, 5] and moss [6]. Besides a role in PPB formation, the TTP complex plays a role in interphase as well, and TTP mutants exhibit strong perturbations in the organization of the interphase cortical array and consequently in oriented expansion. TTP proteins thus have both interphasic and mitotic functions, and the composition, localization and activity of the TTP complex vary significantly during the cell cycle [3]. Our results indeed suggest the existence of a high combinatorial diversity of TTP complexes, as most identified TTP components are present in several isoforms, especially the TRM family which comprises 34 members in Arabidopsis [4].
In the course of our studies, we have identified a large protein network, the TTP complex, controlling the spatial arrangement of cortical MTs both in interphase and at the PPB stage.

The preprophase band (PPB) which consists of microtubules and actin filaments is a land-plant specific cytoskeletal array contributing to the pre-mitotic programming of the position of final cytokinetic plane.
This protein complex not only provides an excellent and unique entry point to better understand transitions of the cortical MT cytoskeleton, but it also allows us to explore regulatory circuits connecting these transitions to the cell cycle.
Over the years, most of our activity has been devoted to the characterization of TTP components like TON1 [1], FASS/PP2A [2, 3] or TRM [4]. Altogether, a remarkably consistent picture of this large protein complex emerges from a number of cytological, molecular, genetic and biochemical evidences.
Mutations in core-TTP components like TON1, FASS or PP2A A and C subunits lead to complete absence of PPB and profound disorganization of division planes in Arabidopsis [1, 2, 3, 5] and moss [6]. Besides a role in PPB formation, the TTP complex plays a role in interphase as well, and TTP mutants exhibit strong perturbations in the organization of the interphase cortical array and consequently in oriented expansion. TTP proteins thus have both interphasic and mitotic functions, and the composition, localization and activity of the TTP complex vary significantly during the cell cycle [3]. Our results indeed suggest the existence of a high combinatorial diversity of TTP complexes, as most identified TTP components are present in several isoforms, especially the TRM family which comprises 34 members in Arabidopsis [4].
Reevaluation of the role of the PPB, and of the importance of division geometry in plant morphogenesis
In a search for (pre-)mitotic isoforms of the TTP complex, we conducted a detailed analysis of the TRM6-7-8 family of TRMs.

We showed that TRM7 displays a very specific expression/localization/degradation profile at the G2/M transition and labels the PPB. PPB formation was completely abolished in the triple trm678 mutant, providing a unique opportunity to assess PPB function independently of the Interphase Cortical Array [7]. Contrary to expectations, PPB-less plants exhibited little morphological defects, and rather showed a general reduction in growth capacity. We showed that PPB absence did not abolish the ability to define the division site at the cortex, but induced a loss of precision in division plane positioning. These results led us to redefine PPB function as a noise-suppression filter rather than a primary determinant as previously thought. Interestingly, PPB-less cells display mispositioned spindles, suggesting that the stabilizing effect of the PPB could stem from a role in confining spindle rotations in prophase/metaphase.
In a search for (pre-)mitotic isoforms of the TTP complex, we conducted a detailed analysis of the TRM6-7-8 family of TRMs.

We showed that TRM7 displays a very specific expression/localization/degradation profile at the G2/M transition and labels the PPB. PPB formation was completely abolished in the triple trm678 mutant, providing a unique opportunity to assess PPB function independently of the Interphase Cortical Array [7]. Contrary to expectations, PPB-less plants exhibited little morphological defects, and rather showed a general reduction in growth capacity. We showed that PPB absence did not abolish the ability to define the division site at the cortex, but induced a loss of precision in division plane positioning. These results led us to redefine PPB function as a noise-suppression filter rather than a primary determinant as previously thought. Interestingly, PPB-less cells display mispositioned spindles, suggesting that the stabilizing effect of the PPB could stem from a role in confining spindle rotations in prophase/metaphase.
Highlighting the strong coupling/signaling between oriented cell growth and division
Our published and unpublished results point to a variety of TTP isoforms, specializing in either cell division, cell elongation or cell differentiation during Arabidopsis development. Interestingly, our studies on various mutations in TTP components, alone or in combination, reveal that in cycling cells, growth and division are tightly spatially coupled through the organization and co-alignment of microtubule arrays during the cell cycle: mutations primarily affecting cell division orientation have a strong impact on cell elongation, and the reverse is true as well. Therefore division, elongation, and differentiation should not be studied separately, and we are developing new approaches using microfluidics, high resolution time-lapse microscopy and image analysis to reveal these connections.
Our published and unpublished results point to a variety of TTP isoforms, specializing in either cell division, cell elongation or cell differentiation during Arabidopsis development. Interestingly, our studies on various mutations in TTP components, alone or in combination, reveal that in cycling cells, growth and division are tightly spatially coupled through the organization and co-alignment of microtubule arrays during the cell cycle: mutations primarily affecting cell division orientation have a strong impact on cell elongation, and the reverse is true as well. Therefore division, elongation, and differentiation should not be studied separately, and we are developing new approaches using microfluidics, high resolution time-lapse microscopy and image analysis to reveal these connections.
Cited Publications
1. Azimzadeh et al. (2008). Plant Cell 20, 2146-2159
2. Camilleri et al. (2002). Plant Cell 14: 833-845
3. Spinner et al. (2013). Nature Com, 4:1863
4. Drevensek et al. (2012). Plant Cell 24: 178-191
5. Traas et al. (1995). Nature 375: 676-677
6. Spinner et al. (2010). Development, 137: 2733-2742
7. Schaefer et al. (2017). Science 356, 186–189
Leader:
David Bouchez