In all organisms, storage lipids are maintained in the cytoplasm in specialized organelles called lipid droplets (LDs). These nanoparticles consist mainly of a core of neutral lipids (triacylglycerols and/or steryl esters) enclosed in a monolayer of phospholipids, and contain a number of proteins which vary considerably with the species.
More than inert fat balls, LDs are complex organelles and their abnormal dynamics is associated with several diseases (virus-induced steatosis, diabetes, atherosclerosis and myopathies).
In the context of green chemistry, LDs are promising sources of lipid-derived molecules for chemistry, food, medicine and cosmetics. For these reasons, understanding LD structure and way of life is of major importance.
Study of the integral class I proteins of the lipid droplet.
We are interested in the structural and functional study of integral class I proteins of LDs inserted in their native context. We are currently studying the evolutionary convergence of these proteins in different kingdoms. Indeed, many proteins associated with the surface of LDs do not have primary amino acid sequence conservation but structural convergence with a hairpin-like folding. This particular folding allows strong anchoring of these proteins within the phospholipid monolayer and contact with the neutral lipid core of LDs.
Few structural data exist on these proteins, as they are very difficult to manipulate due to their high hydrophobicity. No study on this structural convergence exists presently. We have developed a model cell system based on the heterologous expression of these LD class I proteins in Saccharomyces cerevisiae (Purkrtova, 2015, Vindigni et al 2013, Jamme et al. 2013). This tool has allowed us to perform several functional and structural studies of the LD Class I proteins of seeds.
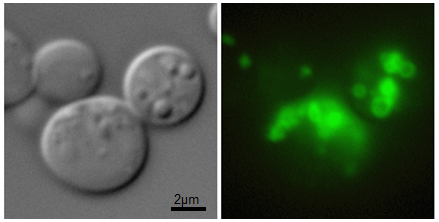
Currently we are interested in other LD class I proteins including caveolin and hepatitis C virus (HCV) core protein. The HCV core protein associates with LDs during infection and participates in the accumulation of LDs in liver cells leading to steatosis which can progress to cirrhosis or cancer. Understanding the targeting and anchoring mechanisms of the core protein, as well as its impact on the metabolism of lipids and LDs in the cell, may favor the development of treatments for HCV infection or other viruses that divert LDs for their viral cycle.
This work is carried out in collaboration with Y.Gohon (INRAE) and is supported by the ANRS grant CI19044.
Structural study of lipid droplet in cellulo using synchrotron label free imaging
The core of lipid droplet consists mainly of neutral lipids, triglycerides and/or sterol esters. Organization of these lipids inside the core is still under debate. Scientists have observed segregation of these lipids but it has been suggested by others that this may be an artifact related to isolation or chemical fixation of LDs. It appears essential to be able to image LDs in their native state and in an unaltered cellular environment. We have developed imaging techniques for the ultrastructural study of native lipid droplets in cellulo.
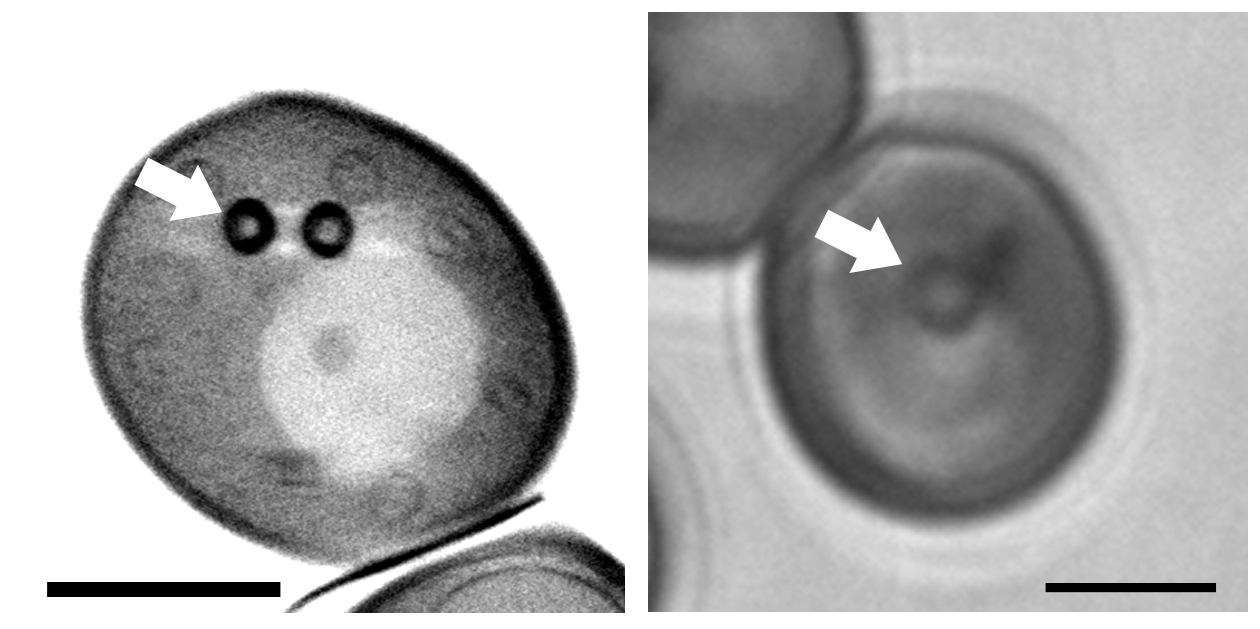 We have shown that LDs are organized structures, with segregation of sterol esters at the periphery of the LD core using label free synchrotron radiation techniques, soft x-ray tomography and deep UV transmittance (Jamme et al. in press). The use of multimodal deep UV imaging, transmittance and tryptophan/tyrosine autofluorescence also provides information on chemical content at the subcellular scale. These multimodal approaches pave the way for the development of new techniques for imaging organelles without labeling.
We have shown that LDs are organized structures, with segregation of sterol esters at the periphery of the LD core using label free synchrotron radiation techniques, soft x-ray tomography and deep UV transmittance (Jamme et al. in press). The use of multimodal deep UV imaging, transmittance and tryptophan/tyrosine autofluorescence also provides information on chemical content at the subcellular scale. These multimodal approaches pave the way for the development of new techniques for imaging organelles without labeling.This work is carried out in collaboration with F. Jamme, M. Réfrégiers (SOLEIL synchrotron), B. Cinquin (IPGG CNRS), E. Pereiro (ALBA synchrotron), Y. Gohon (IJPB, INRAE) and has received financial support from the SOLEIL synchrotron (grants 20140219; 20141082; 20150869) and the ALBA synchrotron (grant 2015021150).
Biodiversity of lipid production in Saccharomycotina
The exploitation of the genetic resource of living organisms is one of the approaches proposed to meet the needs of the green chemistry and nutrition-health sectors for the production of lipids towards molecules that respond usage constraints. Exploratory analyses of the fatty acid content carried out on different yeast species have revealed the presence of highly contrasting profiles between strains.
Biodiversity of lipid production in Saccharomycotina
The exploitation of the genetic resource of living organisms is one of the approaches proposed to meet the needs of the green chemistry and nutrition-health sectors for the production of lipids towards molecules that respond usage constraints. Exploratory analyses of the fatty acid content carried out on different yeast species have revealed the presence of highly contrasting profiles between strains.
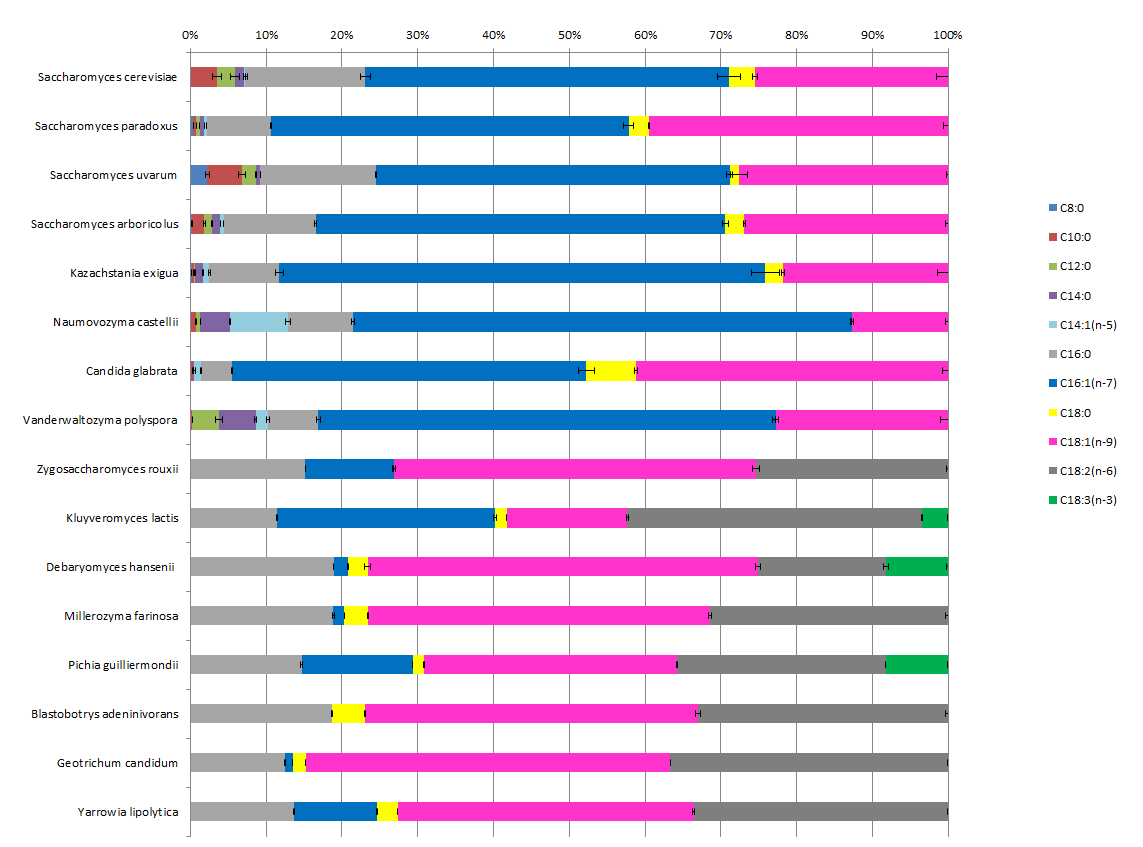 We were able to show that
We were able to show that-
Medium fatty acids are present in a very small number of species (so-called post-WGD, whose ancestor underwent genome duplication) (Froissard, et al. 2015).
-
Very high levels of polyunsaturated fatty acids (PUFAs) are present in some geographical isolates of species of the genus Debaryomyces. Bioinformatic analysis of the presence of certain genes in genome-sequenced yeasts indicates an atypical distribution in agreement with observations on PUFA production.
We are currently focusing on the omega 3 content of a subset of strains and studying the possible relationship between
- Omega 3 content -Cell physiology (morphology, membrane fluidity, ...)
- geographic origin of the strains -allele diversity of the FAD3 gene responsible for the desaturation of omega6 into omega3,
- regulation of these genes.

Contacts
Cell Differentiation and PolarityTél : 0130833896